
Spinal pain is commonly treated with anti-inflammatory drugs. But how effective are they?
Most scientific presentations begin with a PowerPoint slide showing all of the speaker’s conflicts of interest. Not all conflicts of interest are financial. Some can be personal. My conflict of interest today is that I’ve suffered from occasional back pain, on and off, for years. And when I’ve had acute pain, I’ve taken non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen (Advil). And while I’ve never found them to be miracle drugs, I did believe that they were providing some degree of pain relief, albeit with risks. Earlier in my career, I learned just how toxic these drugs can be. Working 12-hour shifts, always on my feet, led to some debilitating back pain. I took NSAIDs daily, for weeks. I was eventually diagnosed with a stomach ulcer. Since that time, I’ve found regular exercise seems to keep the back pain at bay, most of the time. And I’m pretty cautious about taking ibuprofen anymore. But I still use it occasionally, assuming that the potential for benefit outweighed any short-term risk. Now there’s a new study that suggests that the benefits of NSAIDs for spinal pain may be even more modest than I (and others) may have been led to believe. And it’s generating new questions about the best approach to spinal pain.
The ubiquity of spinal pain
Spinal pain, whether it’s low-back pain or neck pain is a common cause of disability and among the most common reasons for physician consultation. While most cases of acute pain are self-limiting and will resolve over time, they can be very debilitating, and I find as a pharmacist that people are willing to do or try just about anything if they think it will help them get back to normal function. Despite how common spinal pain is, the causes are poorly understood, and in most cases, never identified. While heat, ice, physical therapies, and spinal manipulation are sometimes sought out for acute spinal pain, self-managed therapy often starts (and ends) with analgesics and anti-inflammatory drugs. They are the mainstay of therapy. But do they deserve to be?
NSAIDs are among the most widely used drugs in the world, starting in infancy for pain and fever, right through to the elderly where they are standards for treating osteoarthritis and other muscle and skeletal conditions. NSAIDs all work in the same way, blocking the cyclooxygenase (COX) enzyme, responsible for the production of prostaglandin messenger substances that cause pain, inflammation and fever. The mechanism of action is also responsible for the extensive side effect list, a consequence of COX enzymes being distributed throughout the body. Ulcers are the most well-known effect and hospitalization secondary to gastrointestinal bleeding from NSAIDs is common. Fortunately these side effects can be prevented with drugs like omeprazole. The other well-known side effect is cardiovascular disease, and NSAIDs seem to increase the risks of heart attacks and strokes. Given they’re used so frequently for spinal pain conditions, understanding their risk and potential benefit is essential. That’s what a new study set out to document.
This new paper is entitled “Non-steroidal anti-inflammatory drugs for spinal pain: a systematic review and meta-analysis,” and was published in the Annals of the Rheumatic Diseases. The advantage of a systematic review is that it can give more precision and accuracy to a specific clinical question, pulling together the results from multiple smaller trials into a comprehensive analysis. A systematic review looks at all the evidence on a topic, eliminating the risk of arbitrarily “cherry picking” the trials that point to a specific or erroneous conclusion. The risk of a systematic review is that it can be a GIGO scenario, as ultimately the review’s usefulness is a function of the types of studies that went into it. There can be good and bad aspects to these types of reviews, which I’ll come back to.
The investigators (Gustavo C. Machado and colleagues) cast a “wide net” looking for studies – they used fairly broad criteria. They searched the major databases for randomized controlled trials, published in peer-reviewed journals, comparing NSAIDs with placebo for spinal pain. Trials had to include those with neck or back pain, with or without “radicular” pain (radiating pain). They included acute (short-term) and chronic (long-standing) spinal pain, and including any type of NSAID as well as any formulation (oral, injectable and topical forms were included). They excluded patients with serious spinal conditions or other conditions (e.g., cancer) and they also excluded pain management after surgery. All non-randomized trials, guidelines, and observational studies were excluded. I’m not going to describe the data extraction and trial quality assessment process. I am not a methodologist, but the process described looks consistent with other systematic analyses.
Measuring pain and pain relief
A visual analog pain scale
One of the challenges in measuring pain, especially in clinical trials, is that there’s no objective marker. We rely primarily on visual analogue scales (0-100) or asking patients to rate their pain (“How does your pain feel on a scale of 0 to 10”). The image above is an example of a scale that might be used. An important question when using a pain scale is determining what constitutes a “meaningful” difference. This often comes up in the discussion of alternative medicine where a very weak (but statistically significant) difference is shown in some measure, which is cited as “proof” that the intervention (e.g., acupuncture) “works”. We use the term “clinically important” or “clinically relevant” to try to distinguish between changes that are meaningful to patients, and those that are likely not. For example, if you rate your pain a 70 today and a 66 tomorrow, that’s not likely to be clinically meaningful. In this analysis, a difference of 10 points (on a 0-100 scale) is what the authors specified they considered the smallest worthwhile effect.
The results
After searching and screening, only 35 randomized trials were found, a number I found disappointingly low given how frequently NSAIDs are used for the spinal pain. Most of the trials were for oral products, but five looked at intravenous or topical treatments. Twenty-two trials examined low back pain, split evenly between studies of acute and chronic pain. Eleven trials looked at sciatica and only two looked at neck pain only. Amazingly, the median treatment duration in the trials was seven days. While there were a relatively small number of trials, they were of generally high quality. Overall, the researchers assessed that there was a low risk of bias in the results.
Efficacy
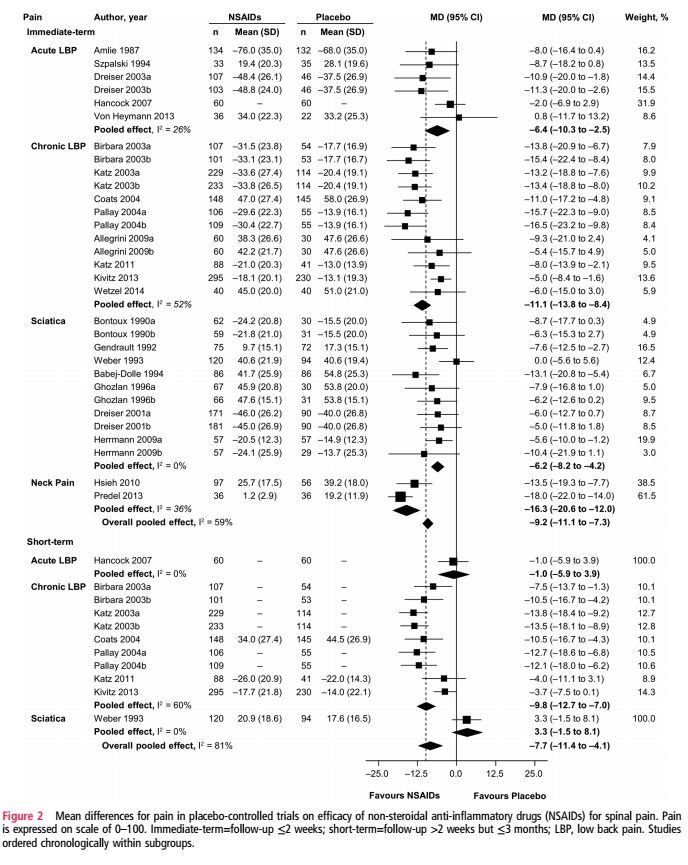
The pooled analysis found moderate-quality evidence that NSAIDs do reduce pain in the short- and intermediate-term, compared with placebo, with mean differences in pain scores of -7.7 and -9.2, respectively. The number needed to treat to obtain clinically significant pain relief effects was calculated at 6 for short-term pain, and 5 for intermediate-term pain. There were also real effects on disability with a short term decrease of -6.1 and intermediate-term effects of -8.1. Given the mean difference between the groups was less than 10 for all measures, the authors noted that these differences were not expected to be clinically important. There were no studies reporting medium- or long-term effects of NSAIDs on spinal pain. Figure 2, which I’ve reproduced below, shows that there’s undeniably a real effect from NSAIDs, with some studies showing substantial effects. At issue is the extent to which we should consider the overall effectiveness to be clinically meaningful:
Safety
Twenty-one trials were included in the safety analysis, where the median treatment duration was 7 days. There were no differences noted in “any event” adverse events reports between NSAIDs and placebo. However, when looking specifically at gastrointestinal effects, there were more adverse events reported in the NSAID group, with a relative risk of 2.5 compared with placebo. This isn’t surprising. I’ve blogged before about the overall safety profile of NSAIDs, which wasn’t the subject of this study.
Use of “rescue” medications
It’s not uncommon in pain trials to look at the use of other analgesics as a proxy for the effectiveness of the treatment. The use of “rescue” analgesics was studied in a pooled analysis of 4 trials, and while there was a modest (yet statistically significant) reduction (-0.4 tablets/day) in the NSAID group, the authors comment that this is “arguably not clinically significant.”
Secondary analyses
The authors sliced and diced the studies and found:
- Trials with the lowest risk of selection bias had larger effects (mean difference -11.2)
- COX-2 drugs (like Celebrex) had larger effects than the “nonselective” NSAIDs (mean difference -13.4 vs -7.7)
- There were slight differences in the effects of the different dosage forms (topical MD -13.2, oral MD -8.5, injectable MD -9.5)
Conclusion: NSAIDs reduce pain…somewhat
NSAIDs reduce spinal pain. This effect is observed across different drugs and even with different dosage forms. The effects are modest, however, and the overall usefulness is not as obvious as we’d like. Translating the effect size into a number needed to treat, the authors calculated that for every 5-6 people that take NSAIDs for spinal pain, only one will derive clinically meaningful benefits. This is at the expense of a slight increase in the risk of short-term gastrointestinal adverse effects. There are some limitations in the analysis which are driven by the underlying data. Remarkably, despite how common spinal pain is, there’s a startling lack of good evidence to inform our decision-making. There were few trials on neck pain and none on whiplash. While there’s better evidence with low back pain, the short-term nature of the trials leaves open the question about the benefits (if any) of extended usage.
What this paper also reinforces is that we lack proven safe and effective medical treatment options for spinal pain. There’s little evidence that acetaminophen (Tylenol) has any meaningful effects for back pain. Opiate analgesics are not preferred owing to their side effect profile and the risk of dependency. And now we have an analysis suggesting that the effectiveness of NSAIDs is more limited than we might expect. The toxicity of NSAIDs must be considered too. Everyone that takes an NSAID raises their risk of cardiovascular and gastrointestinal harm to some extent. Consequently, the safest strategy is to take the lowest effective dose for the shortest duration of time. Given the maddening “black box” of spinal pain, where the underlying cause is often not understood, perhaps it’s not surprising that there is no panacea to be found in anti-inflammatory drugs.
Photos via Flickr users mzuckerm and Tony Hall used under a CC license.
